Deuterated solvent effects in the kinetics and thermodynamics of keto-enol tautomerization of ETFAA
(1) BASIS Independent Silicon Valley, San Jose, California, (2) Mission San Jose High School, Fremont, California, (3) Department of Chemistry, Biochemistry & Physics, Aspiring Scholars Directed Research Program, Fremont, California
https://doi.org/10.59720/21-115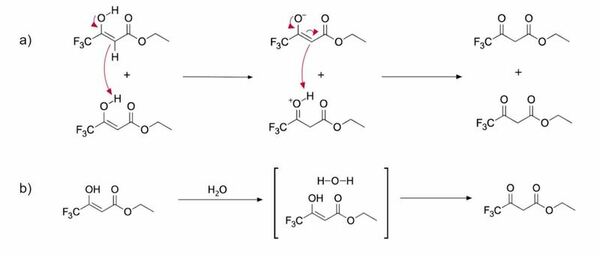
Ethyl 4,4,4-trifluoroacetoacetate (ETFAA) is a β-ketoester that is used as a starting material in the synthesis of a variety of biologically active compounds. The reactivity of this compound is solvent dependent, and here we investigate the kinetics and thermodynamics of keto-enol tautomerization of ETFAA through benchtop 19F NMR in both non-deuterated and deuterated solvents. We determined that tautomerization dynamics in protic and aprotic solvents displayed differences in reaction rates and in the proportion of the keto and enol tautomers present. Moreover, our results demonstrated a positive correlation in aprotic solvents between solvent polarity and the proportion of keto tautomer present. We further employed density functional theory (DFT) calculations to probe the specific chemical interactions responsible for these observed trends. From the DFT calculations it was found that the major source of stability of the enol tautomer compared to the keto tautomer was its intramolecular hydrogen bond. Knowing the equilibrium of the keto-enol tautomerization of ETFAA is vital for synthesis of biologically active compounds derived from ETFAA, and maintaining a computational perspective can be useful in determining the advantages and disadvantages of various computational methods used in modeling keto-enol tautomerization.
This article has been tagged with: