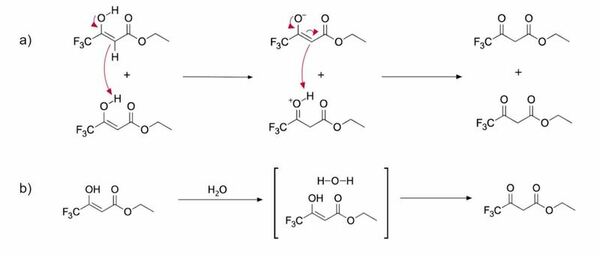
In this study, the authors determined whether tautomerization dynamics in protic and aprotic solvents displayed differences in reaction rates and in the proportion of the keto and enol tautomers present.
Read More...Deuterated solvent effects in the kinetics and thermodynamics of keto-enol tautomerization of ETFAA

In this study, the authors determined whether tautomerization dynamics in protic and aprotic solvents displayed differences in reaction rates and in the proportion of the keto and enol tautomers present.
Read More...Effects of Temperature on Hand Sanitizer Efficiency
Here, recognizing the widespread use of hand sanitizers, the authors investigated their effectiveness in relation to storage temperature. They applied hand sanitizer before and after touching a cell phone and used LB-agar plates to monitor the growth of bacteria following this process. They found that 70% ethyl-alcohol-based sanitizers are least effective at temperatures above 107.27 °F and most effective at 96.17 °F.
Read More...Linear and non-linear summation of responses to visual and olfactory cues in male Drosophila melanogaster
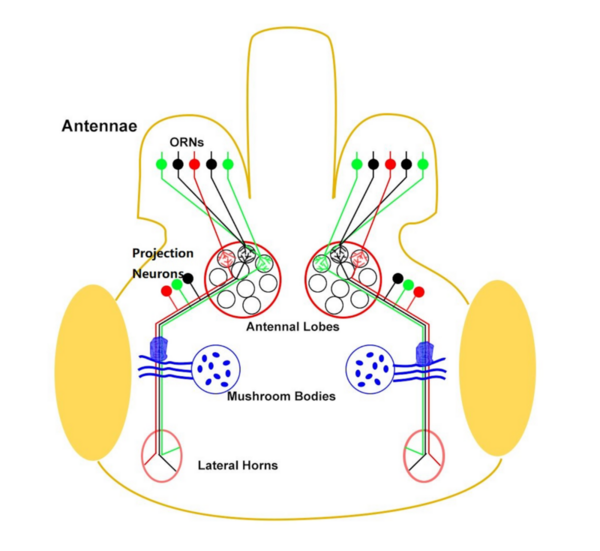
In this study, the authors investigate whether phototaxis and odortaxis in Drosophila melanogaster occurs through linear summation of cues including light and attractive odorants.
Read More...Kinetic Monitoring and Fourier-Transform Infrared (FTIR) Spectroscopy of the Green Oxidation of (-)-Menthol to (-)-Menthone
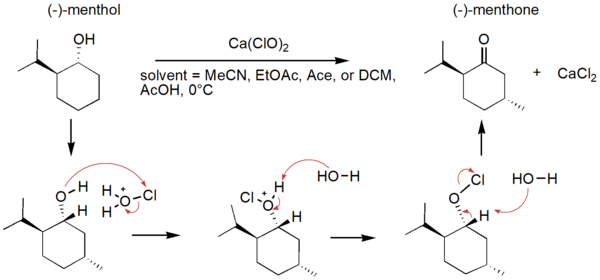
In an effort to reduce the production of hazardous substances, green chemistry aims to make chemical processes more sustainable. One way to do so is changing solvents in chemical reactions. Here, authors assessed different “green” solvents on the oxidation of (-)-menthol to (-)-menthone using Fourier-transform infrared (FTIR) spectroscopy, optimizing the solvent system for this reaction.
Read More...Covalently Entrapping Catalase into Calcium Alginate Worm Pieces Using EDC Carbodiimide as a Crosslinker.
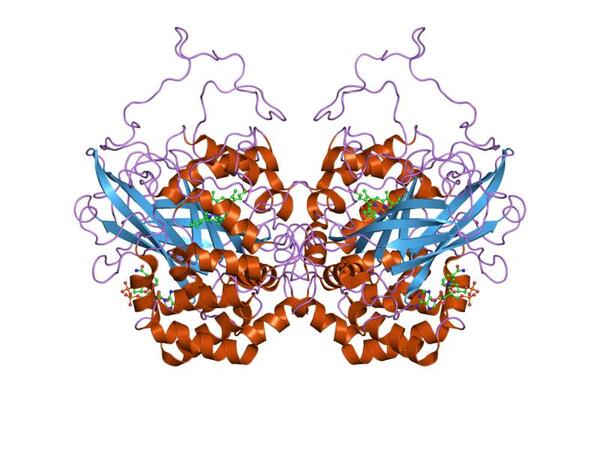
Catalase is a biocatalyst used to break down toxic hydrogen peroxide into water and oxygen in industries such as cheese and textiles. Improving the efficiency of catalase would help us to make some industrial products, such as cheese, less expensively. The best way to maintain catalase’s conformation, and thus enhance its activity, is to immobilize it. The primary goal of this study was to find a new way of immobilizing catalase.
Read More...The Mount Laurel doctrine: A case study in housing affordability and the labor market in New Jersey

The authors explored the effects of the Mount Laurel Doctrine on housing affordability, unemployment rate, and civilian labor force in Burlington County, New Jersey compared to nearby counties.
Read More...