Structure-activity relationship of berberine and G4 DNA reveals aromaticity’s effect on binding affinity
(1) Langley High School, McLean, Virginia, (2) Dougherty Valley High School, San Ramon, California, (3) Henry M. Gunn High School, Palo Alto, California, (4) Abraham Lincoln High School, San Francisco, California, (5) Homestead High School, Cupertino, California, (6) Oswego East High School, Owego, Illinois, (7) Aspiring Scholars Directed Research Program, Fremont, California
https://doi.org/10.59720/22-040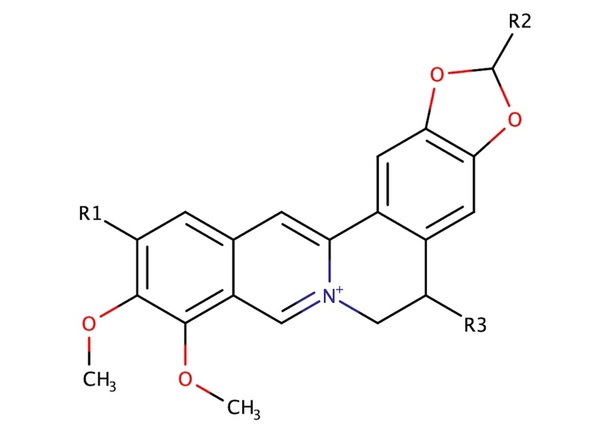
Secondary nucleic acid structures, such as the Guanine Quadruplex (G4), are known to contribute to gene regulation throughout the genome. In cancer cells, stabilizing such structures could prove to be integral to inhibiting cancer cell proliferation. Berberine is a natural quaternary alkaloid with a number of medicinal properties, including antimicrobial and anticancer effects. Previous studies have shown that berberine can stabilize the G4 through π-π interactions, by increasing the release of free energy. In this study, we test the effectiveness of ligand aromaticity (cyclic, planar molecules) on the free energy of binding with the G4. Based on previous studies, we hypothesized that large aromatic rings incorporated in berberine molecules would act as highly stabilizing ligands that would generate the greatest binding affinity with the G4 through π interactions with guanine endplates and K+ ions. To explore the structure-activity relationship for berberine-based ligands, we developed an in silico library of 800+ ligands. Through molecular docking, we predicted the free energy release of each ligand’s interaction with the G4 complex. From our research, we found that berberine analogs with aromatic R groups, which allow for high degrees of aromaticity and flexibility, have a significant positive impact on binding strength between berberine analogs and G4 complexes through π-π interactions with endplates and grooves, indicating that these ligands can more effectively bind to and stabilize the G4’s activity. The authors envision that this research may aid the development of drugs that target the G4 to inhibit cancer cell proliferation, ultimately furthering cancer therapeutics research.
This article has been tagged with: