Racemic serine is less soluble than pure enantiomers due to stronger intermolecular hydrogen bonds
(1) Monta Vista High School, Cupertino, California, (2) Department of Biomolecular Engineering, University of California, Santa Cruz, Santa Cruz, California
https://doi.org/10.59720/21-122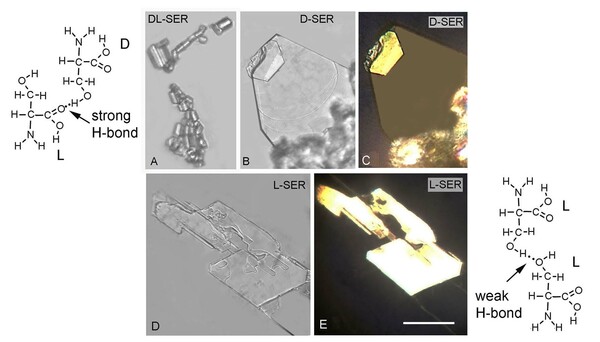
The chemical and physical properties of amino acids determine the properties of the proteins they compose. Two of these properties include solubility and chirality. It is known that pure D- and L-serine are approximately eight times more soluble than DL- serine, their racemic mixture, but the reason is not clear. Our hypothesis is that the difference is related to the types of H-bonds that stabilize crystals of the pure serine enantiomers compared to their DL-racemic mixture. We tested the hypothesis by examining the structures of microscopic crystals and observed that the D- and L-serine formed platelets while the DL- serine formed prisms. We also discovered that when saturated solutions of D- and L-serine are mixed, DL- serine crystals form within seconds, accompanied by release of heat energy corresponding to -2.7 kcal/mol. This exothermic heat can be accounted for if a new type of hydrogen bond can form between the D- and L-serine enantiomers during crystallization that is absent in crystals of pure enantiomers. We confirmed this conclusion by examining crystal structures previously reported in X-ray diffraction studies of serine crystals. The significance of our observation is that release of heat energy during crystallization of a mixture of pure D and L enantiomers has not been previously reported and may be unique to the amino acid serine.
This article has been tagged with: