On the Relationship Between Viscosity and Surface Tension
(1) Hillfield Strathallan College, Hamilton, Canada, (2) McMaster University, Hamilton, Canada, (3) McMaster University, Hamilton, Ontario, Canada
https://doi.org/10.59720/14-024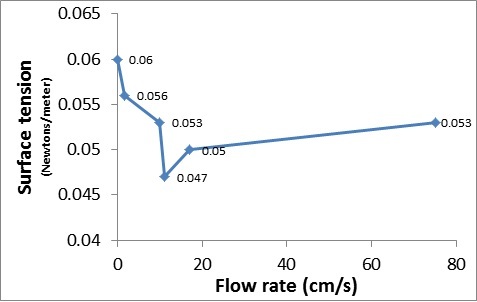
Flow rates and pulling forces were measured in several solutions to determine the correlation between surface tension and viscosity. Because these fluid properties arise from intermolecular bonding, a positive correlation was expected. To study the relationship between viscosity and surface tension, solutions with different concentrations of agar and flour were used. Differences in viscosity were determined by differences in flow rate. The flow rates were determined from the time that the solutions took to flow through a tube. The surface tension (Newtons/meter) was determined by the pulling force exerted on a needle placed on the surface of the solution, which was weighed using grains of rice put on a scale. Surprisingly, we found that the solutions with a higher viscosity than water had either less or the same surface tension as water, and we suspect this is due to the unchanged intermolecular bonding of water molecules (hydrogen bonding) causing surface tension as viscosity increased. A possible explanation is that the viscosity of a fluid is influenced more by the friction caused by the interactions between large molecules with a lot of polar atoms, causing attraction between them.
This article has been tagged with: