Kinetic Monitoring and Fourier-Transform Infrared (FTIR) Spectroscopy of the Green Oxidation of (-)-Menthol to (-)-Menthone
(1) The Quarry Lane School, Dublin, California, (2) Mission San Jose High School, Fremont, California, (3) American High School, Fremont, California, (4) Irvington High School, Fremont, California, (5) Dougherty Valley High School, San Ramon, California, (6) Department of Chemistry, Biochemistry & Physics, Aspiring Scholars Directed Research Program, Fremont, California
https://doi.org/10.59720/20-058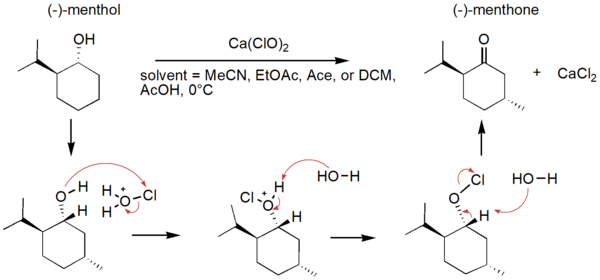
Green chemistry involves reducing the production of hazardous substances in chemical processes to be more sustainable, thereby decreasing pollution and other environmental damage. Solvent is a major contributor of waste in organic synthesis, so the greenness of a chemical reaction can be improved by changing the solvent. The oxidation of (-)-menthol to (-)-menthone in different solvent systems was monitored using Fourier-transform infrared (FTIR) spectroscopy. Our study conducted this reaction in solvent systems of acetic acid and other solvents as replacements for acetonitrile: acetone, ethyl acetate, and dichloromethane. Solvent choice was determined based on the principles of green chemistry, and kinetics and yield of the reactions in different solvents were investigated. Through FTIR spectroscopy, the products of all the reactions were characterized as (-)-menthone. Since a limiting factor in hypochlorite-mediated oxidations of alcohols to carbonyls is the solubility of the hypochlorite salt, we hypothesize that the most polar solvent systems will give the fastest reaction rate and highest yields. Our hypothesis was incorrect, as the reaction time was shortest and product yield was greatest for the oxidation performed in ethyl acetate and acetic acid, which are less polar than acetonitrile. The green oxidation of (-)-menthol to (-)-menthone was optimized in this solvent system.
This article has been tagged with: