A computational quantum chemical study of fluorinated Allopurinol
(1) A K High School, Dhaka, Bangladesh, (2) East-West University, Dhaka, Bangladesh
https://doi.org/10.59720/21-235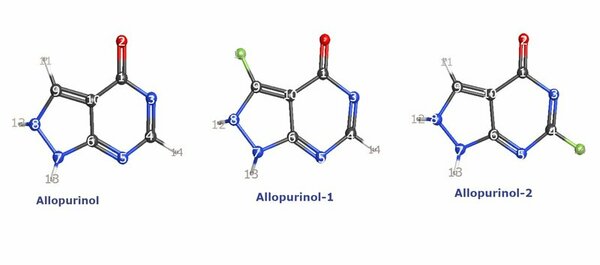
Allopurinol is a two-ring heterocyclic drug. Allopurinol has several conveniences for medicinal chemistry, including easy preparation methods and low toxicity, which make it a preferable choice for researchers and scientists. However, it has some limitations such as a short half-life and low stability. Fluorination is one of the most effective ways to enhance a number of chemical properties and applications of a compound. The small and highly electronegative features of fluorine have thus made it a profitable choice to optimize a compound. The inclusion of fluorine atoms can improve the effectiveness and reduce the limitations of allopurinol was the presumption of this study. From that context, this research deals with an examination of the effect of fluorine atoms on allopurinol. We investigated several chemical properties of allopurinol with quantum chemistry calculations using Hartree-Fock theory. We evaluated the molecular geometry, Mulliken charge analysis, and frontier molecular orbitals calculations. We conducted a comparison of our calculated properties to determine the advantages and disadvantages of allopurinol and two simulated, fluorinated, hybrid compounds. We found higher chemical stability in the hybrid compounds. The first hybrid compound was more organic agent-friendly, whereas the second one was more ionic and polar agent-friendly. The fluorinated compounds showed their potential to prevent metabolic attacks and improve reactivity with different chemical agents.
This article has been tagged with: