AI-designed mini-protein targeting claudin-5 to enhance blood–brain barrier integrity
(1) Hillsdale High School, (2) University of California
https://doi.org/10.59720/25-088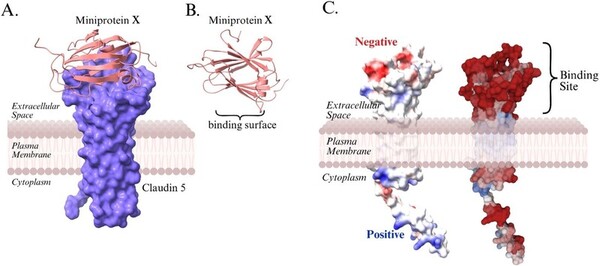
The blood–brain barrier (BBB) is a highly selective interface composed of endothelial cells, astrocytes, and pericytes that maintains central nervous system (CNS) homeostasis and guards against circulating toxins and pathogens in the brain. Disruption of the BBB has been associated with several neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. Among the junctional components that maintain the BBB integrity, claudin-5 (CLDN5) is the principal endothelial tight-junction tetraspan transmembrane protein that maintains paracellular permeability, and its dysregulation is present across multiple neurodegenerative diseases, thereby associating BBB breakdown with specific tight-junction alterations. We hypothesized that strengthening the tight junctions of the BBB by enhancing CLDN5 binding affinity using a computationally designed mini-protein could counteract the disruption of CLDN5. We have performed computational simulations and machine learning techniques to identify the mini-protein capable of binding the CLDN5 protein and tightening the BBB. The CLDN5 and mini-protein structures were predicted via AlphaFold 3. As an extracellular β-barrel transport protein with exceptional ligand-binding capacity Lipocalin-1 (LCN1) was selected and computationally mutated to generate a library of mini-proteins. The mini-proteins were docked on CLDN5 utilizing the HDOCK2.0 software, and the resulting mini-protein-bound CLDN5 structures were analyzed. The mini-proteins were computationally evaluated based on the binding energy formed between the CLDN5 and mini-proteins and the accessible binding location with CLDN5. In addition, hydrogen bonds formed between the mini-protein and CLDN5 were analyzed to ensure reliable CLDN5 binding with the mini-protein and to minimize the likelihood of protein complex dissociation. Considering binding strength, hydrogen bond analysis, and accessible binding location, mini-protein X was the most suitable candidate for further investigation. This research highlights the potential of computationally engineered mini-proteins to stabilize BBB integrity and slow the progression of neurodegenerative diseases.
This article has been tagged with: