Immunogenicity of Minhai 13-valent pneumococcal polysaccharide conjugate vaccine in experimental mice
(1) Beijing No. 8 Middle School, (2) R&D Center of Shandong Yidu Biotechnology
https://doi.org/10.59720/24-213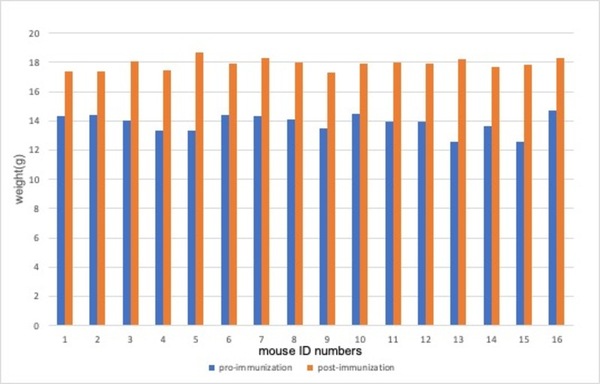
Diseases caused by pneumococci are a global public health problem. The widespread use of antibiotics has led to a sharp increase in the drug resistance of pneumococci, making research on pneumococcal vaccines highly relevant. Pfizer's 13-valent pneumococcal conjugate vaccine (PCV13) is effective; however, its use in low- and middle-income countries is challenged by cost and logistical difficulties in vaccine transportation, storage, and management. Therefore, there is an urgent need to develop efficient, convenient, and affordable vaccines in these countries. An alternative pneumococcal vaccine is produced by Minhai, but its effectiveness is not well understood compared to Pfizer’s vaccine. Differences in bacterial strains, vaccine carrier proteins, and conjugation processes may lead to different immune protections against pathogenic pneumococci. We hypothesized that the pneumococcal conjugate vaccine produced by the Minhai company would elicit significant immunogenicity in experimental mice, though potentially lower than that of the Pfizer vaccine. To test this hypothesis, we gave the Pfizer or Minhai vaccine to mice. Then we used an enzyme-linked immunosorbent assay (ELISA) to detect the antibody geometric mean titer (GMT) of serum IgG antibodies in the vaccinated mice. Although there were slight differences in the antibody titers against the 13 serotypes, these differences were not statistically significant, and the antibody titers for all serotypes met the requirements for immune protection. We concluded that the 13-valent pneumococcal conjugate vaccine produced by the Minhai company elicits a robust immune response in mice.
This article has been tagged with: