In vitro dissolution and in vivo response of pseudoephedrine dosage forms
(1) Westwood High School, (2) XBiotech USA Inc.
https://doi.org/10.59720/24-170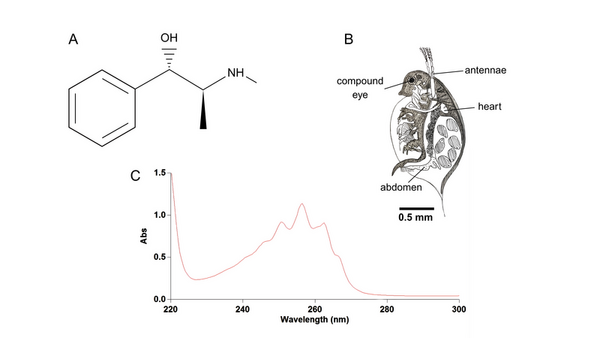
The pharmacological activity of therapeutic oral drugs depends on the speed of release, location within the gastrointestinal tract, and time needed for absorption into the bloodstream. Moreover, the study of the relationship between in vitro dissolution and in vivo pharmacological response is critical, and there is an increasing interest in designing relevant in silico models. Pseudoephedrine HCl is a nasal decongestant with a side effect of increased heart rate. Our research looked at the relationship between pharmacokinetics of various forms of pseudoephedrine HCl and the pharmacodynamic response on Daphnia magna. We hypothesized that drug release kinetics in vitro may not correlate directly with the in vivo response. Our study was comprised of two-parts: i) comparing dissolutions of immediate-release, 12-hour, 24-hour, and enteric-coated pseudoephedrine formulations in gastric and intestinal environments, and ii) evaluating the effect of released pseudoephedrine HCl on heart rate. Our results revealed that maximum drug release was at 55 minutes for the immediate-release form, ~4 hours for the 12-hour release form, and ~18 hours for the 24-hour release form at the two pHs tested. The enteric-coated capsules released only at pH 7.2. There was a positive correlation between drug release and heart rate, with a 36% increase at 120 minutes. Although the drug dissolution kinetics from the immediate-release tablet reached its maximum at 55 minutes, the maximum in vivo response occurred at 120 minutes. Therefore, in vitro-in vivo correlation studies could help in the development of comprehensive in silico models capable of predicting pharmacological parameters for novel drugs in the future.
This article has been tagged with: