Nonthermal nitrogen fixation with air and water by using a low-pressure plasma
(1) METU DF High School, (2) Chemistry Department, METU DF High School, (3) Chemical Engineering Department, Middle East Technical University
https://doi.org/10.59720/24-125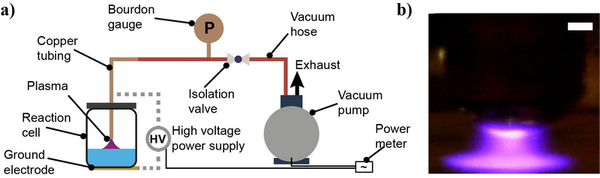
In the 20th century, the increasing demand for food was addressed successfully by establishing the production of synthetic fertilizers through the Haber-Bosch (HB) process. The HB process utilizes atmospheric nitrogen and hydrogen derived from steam-methane reforming to produce ammonia, which can be transformed into common fertilizers like ammonium nitrate and urea. Despite being energy efficient, the high carbon dioxide footprint of the HB process has led researchers to seek alternative routes for generating small nitrogenous compounds. One of the candidates is using plasma technology, which is generally limited by its energy requirements and small plasma size at atmospheric pressure. We hypothesized that when a nonthermal air plasma is generated over water under vacuum, it could improve nitrogen fixation rates and energy efficiency due to the formation of a larger plasma and the production of more water vapor. At a pressure of approximately 160 Torr, we observed that a high concentration of nitrate, a considerably small amount of nitrite, and ammonium were formed in water in a plasma-liquid cell. We investigated the effect of plasma activation time and the level of total dissolved solids on the amount of fixed nitrogen species and energy efficiency. When compared with literature data, our results suggest that operation under vacuum may increase the feasibility of plasma nitrogen fixation, constituting a step towards the production of synthetic fertilizers without carbon dioxide emissions.
This article has been tagged with: