Exploring the possibilities for reactions between SiW and alkaline solutions to be renewable energy sources
(1) Kang Chiao International School, (2) American School of The Hague
https://doi.org/10.59720/23-036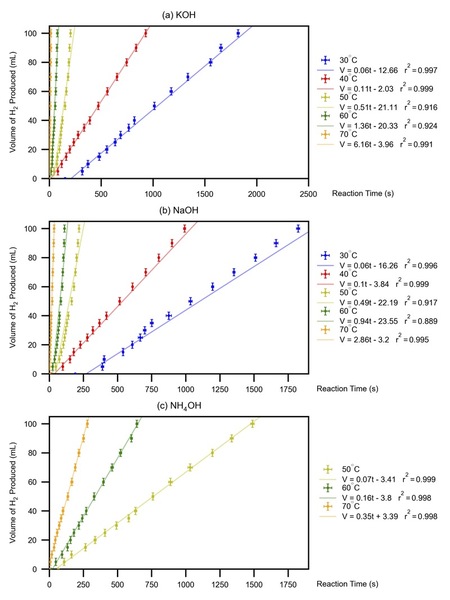
The increasing concern over greenhouse gas emissions (GGE) has led to a search for cleaner energy sources, with hydrogen fuel cells being a promising alternative to fossil fuels. This study focused on the reaction between excess silicon (Si) and limiting alkaline solutions to produce hydrogen (H2) gas, which can be consumed in an electrochemical cell to generate renewable energy. We investigated the effects of reaction temperature, concentration, and alkaline solution type on H2 production rate because these variables are known to influence reaction kinetics and yield. By analyzing the correlation between H2 production and reaction time and deriving the average H2 production rate, we aimed to identify the most efficient conditions for H2 generation. From the best-fit line of the volume-time profile, we deduced the average H2 production rate. The results showed that higher temperatures, higher concentrations, and solutions with higher base dissociation constants (kb) lead to a higher H2 production rate. Along with this finding, the stable and consistent production of H2 gas throughout all the trials demonstrated the viability of using Si waste as a source of renewable energy through hydrogen fuel cells.
This article has been tagged with: