Determining degree of dissociation through conductivity
(1) Milton Academy, (2) Department of Science, Milton Academy
https://doi.org/10.59720/22-286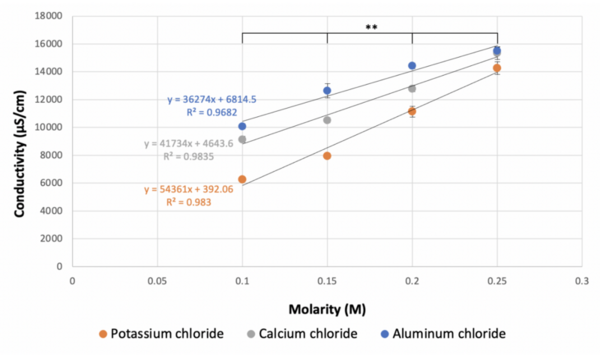
When ionic compounds dissolve in a polar solvent, they dissociate into separate ions and spread uniformly throughout the solution. The greater the degree of dissociation of an ionic compound, the greater the conductivity of the solution will be. The goal of this experiment was to assess how the degree of dissociation of an ionic compound is impacted by the molarity of the compound in solution, the magnitude of the charge of the ion, and the number of ions it produces upon dissociation. Conductivity was measured as a quantifiable representation of the degree of dissociation. We hypothesized that as the concentration of ions in the solution decreased, the conductivity of the solution would also decrease due to fewer interactions between the ions, resulting in a weaker flow of electrical current. We tested our hypothesis by measuring the conductivity of 0.25 M, 0.20 M, 0.15 M, and 0.10 M solutions of aluminum chloride, potassium chloride, and calcium chloride. The data showed that as the molarities of the solutions decreased, the conductivities of the solutions decreased. We also found that the conductivity of each solution did not decrease by the theoretical factor predicted by the number of ions each compound produced upon full dissociation, leading us to conclude that the impact of the charge magnitude of the ions on the degree of dissociation of the ionic compounds must also be considered.
This article has been tagged with: