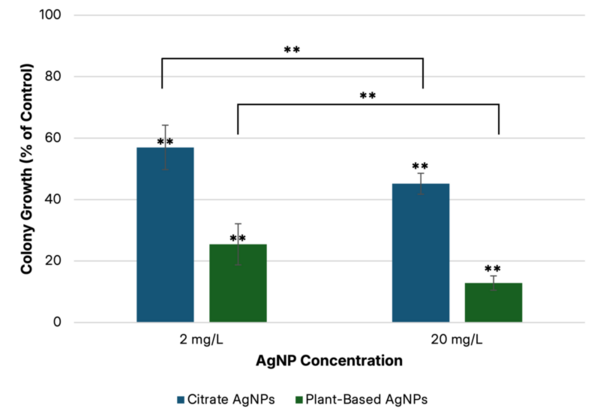
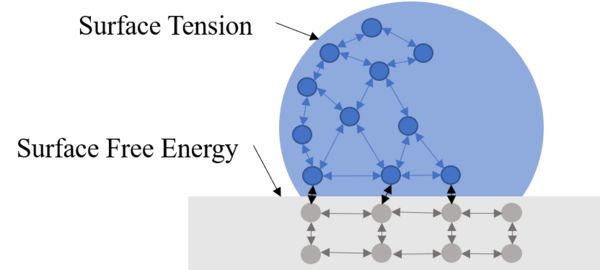
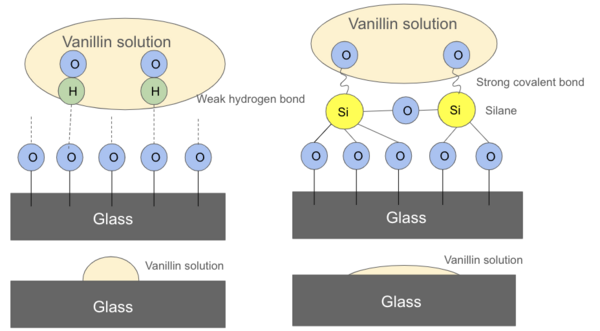
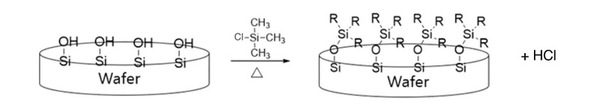
In the aerospace industry, various surface processing or coatings are widely used. However, no detailed research on the aerospace fastener tensile and double shear strength variation due to surface processing has been conducted. Thus, the purpose of this study was to systematically evaluate the effect of surface processing on the standard aerospace fastener's tensile and shear properties.
Read More...