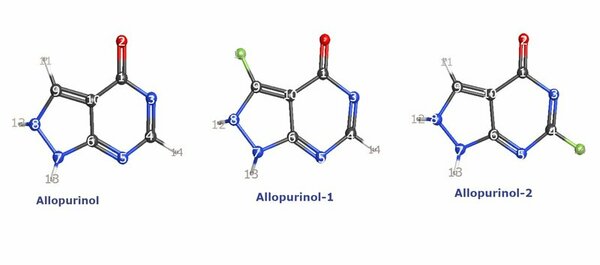
In this study, the impact of fluorination of heterocyclic drug Allopurinol on its stability and other chemical properties is investigated.
Read More...A computational quantum chemical study of fluorinated Allopurinol

In this study, the impact of fluorination of heterocyclic drug Allopurinol on its stability and other chemical properties is investigated.
Read More...Willingness to visit the pediatric dentist during the COVID-19 pandemic

Because of the COVID-19 pandemic, people are missing important appointments because they are viewed as nonessential, possibly including children's pediatric dentist appointments. This study aims to determine how the COVID-19 pandemic has effected parents' willingness to allow children to visit pediatric dental practices and what safety measures would make them feel more comfortable visiting the dentist. The authors found a weak positive correlation between parents' unwillingness to allow their child to visit the dentist, however overall anxiety towards visiting the dentist during the pandemic was low.
Read More...Prediction of molecular energy using Coulomb matrix and Graph Neural Network
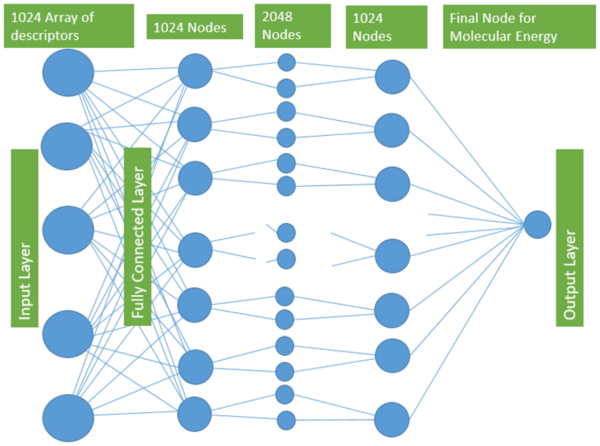
With molecular energy being an integral element to the study of molecules and molecular interactions, computational methods to determine molecular energy are used for the preservation of time and resources. However, these computational methods have high demand for computer resources, limiting their widespread feasibility. The authors of this study employed machine learning to address this disadvantage, utilizing neural networks trained on different representations of molecules to predict molecular properties without the requirement of computationally-intensive processing. In their findings, the authors determined the Feedforward Neural Network, trained by two separate models, as capable of predicting molecular energy with limited prediction error.
Read More...Developing “Off the Shelf” Pancreases for Diabetic Patients Using Bacterial and Kombucha Tea Waste
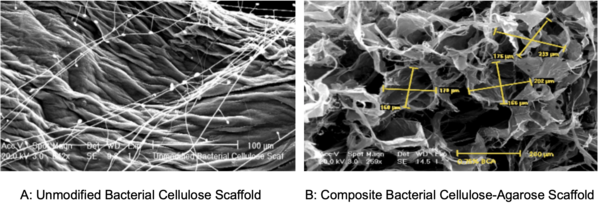
In this study, the authors investigate the suitability of using bacterial cellulose as a scaffold for cell transplants. Interestingly, this cellulose is a can be found in the discard from a symbiotic culture of bacteria and yeast (SCOBY) used to make kombucha.
Read More...Characterization of Inflammatory Cytokine Gene Expression in a Family with a History of Psoriasis
Psoriasis is a heritable autoimmune disorder characterized by abnormal red and itchy skin patches. The authors study the family of a man with psoriasis. They explore whether the man's children, who do not show any symptoms of psoriasis, demonstrate gene expression consistent with the disease.
Read More...Phytoplankton Plastid Proteomics: Cracking Open Diatoms to Understand Plastid Biochemistry Under Iron Limitation
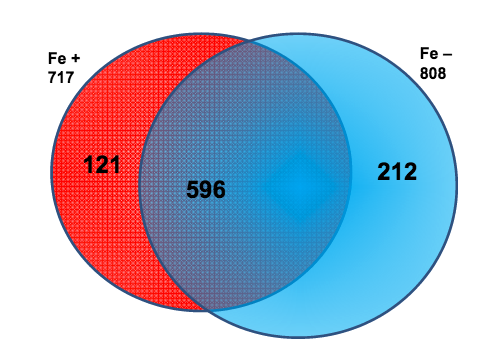
In many areas of the world’s oceans, diatoms such as Thalassiosira pseudonana are limited in growth by the availability of iron (Fe), which is an essential nutrient for diatoms. The authors of this study examined if Fe-limitation makes a significant difference in the proteins expressed within the chloroplast, the power source for diatoms, utilizing a new plastid isolation technique specific to diatoms and completing 14 mass spectrometry experiments.
Read More...Does Music Directly Affect a Person’s Heart Rate?
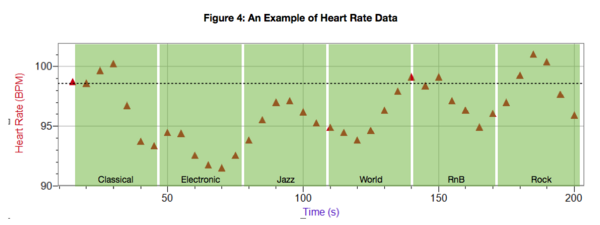
People react to music by moving and dancing. This study examined if different types of music were correlated with higher heart rates and if this was at all affected by music preferences.
Read More...Evaluating TensorFlow image classification in classifying proton collision images for particle colliders
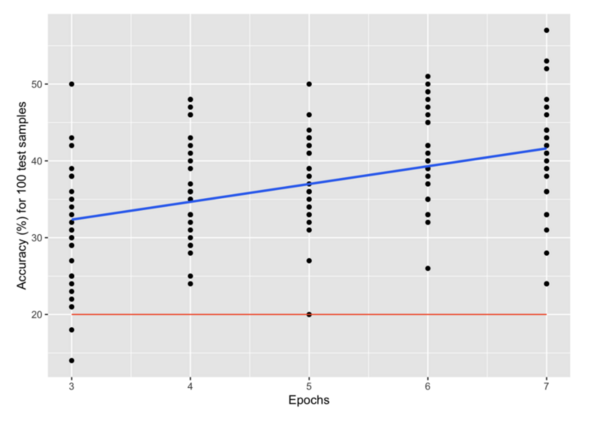
In this study the authors looked at developing a more efficient particle collision classification method with the goal of being able to more efficiently analyze particle trajectories from large-scale particle collisions without loss of accuracy.
Read More...Novel environmentally friendly approach to wastewater treatment eliminates aluminum sulfate and chlorination

The authors tested environmentally-friendly alternatives to wastewater treatment chemicals, including activated charcoal for filtration and citrus peels for preventing bacterial growth.
Read More...Taft linear free-energy relationships in the biocatalytic hydrolysis of sterically hindered nitrophenyl ester substrates
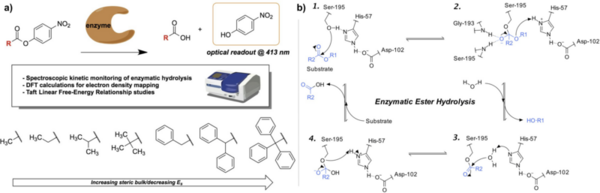
This study applies Taft linear free-energy relationships to study kinetic trends in the enzymatic hydrolysis of sterically hindered substrates.
Read More...