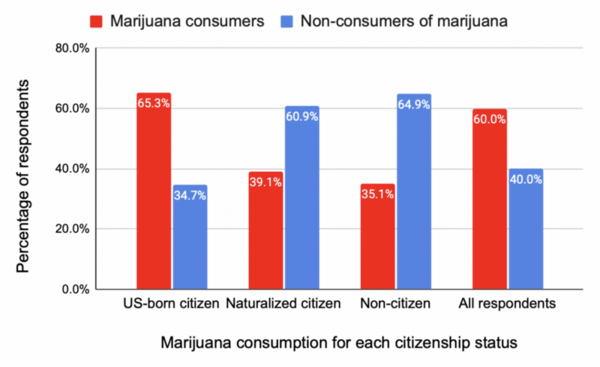
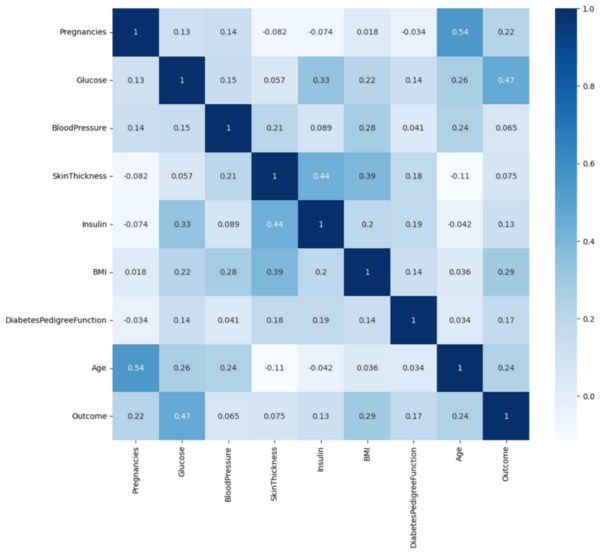
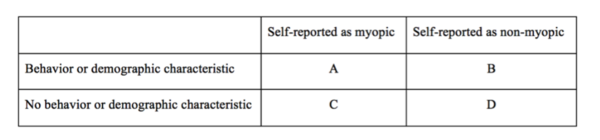
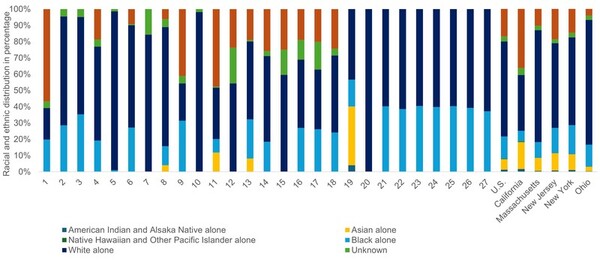
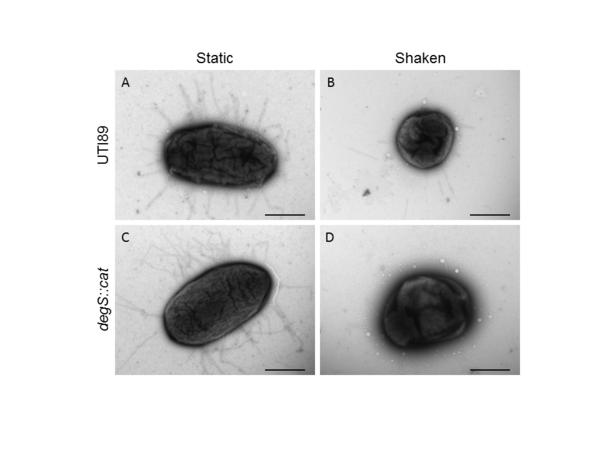
.jpg)
The major drawback of chemotherapy regimens for treating cancer is that the cancerous cells acquire drug resistance and become impervious to further dose escalation. Keeping in mind the studied success of herbal formulations with regard to alternative treatments for cancer, we hypothesized that the use of a chemotherapeutic drug and proprietary herbal formulation, HF1, would combat this phenomenon when administered with common chemotherapeutic drug 5FU. Results demonstrated a cooperative effect between HF1 and 5FU on the drug resistant cell line, implying that administration of HF1 with 5FU results in cell death as measured by MTT assay.
Read More...