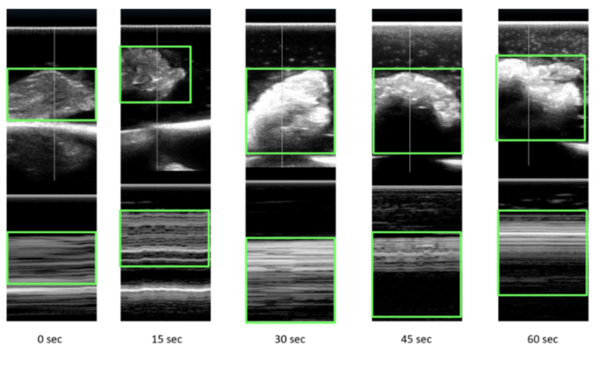
Microwave energy (ME) is used in the medical field to denature protein structures, resulting in inactivation or destruction of abnormal cells. Identifying the extent of destruction of abnormal tissue (cancer tissue or tissue with abnormal electrical activity) is essential for accomplishing successful therapy and reducing collateral damage. Our study was an ex vivo assessment of the changes on ultrasound scans (US) in chicken tissue exposed to ME. We hypothesized that any changes in tissue structures would be recognized on the reflected ultrasound waves. Ultrasound scans of tissues change with exposure to microwaves with increasing reflection of ultrasound waves. With exposure to microwaves, surface level brightness on the ultrasound scans increases statistically significantly. The findings could be used in heat related (ME and radiofrequency) procedures where clinicians would be able to actively assess lesions in real-time. Further studies are required to assess changes in tissue during active exposure to different types of energies.
Read More....jpg)