Pressure and temperature influence the efficacy of metal-organic frameworks for carbon capture and conversion
(1) Thomas Jefferson High School for Science and Technology, Alexandria, Virginia, (2) Division of Pre-college and Undergraduate Programs, Brown University, Providence, Rhode Island
https://doi.org/10.59720/22-244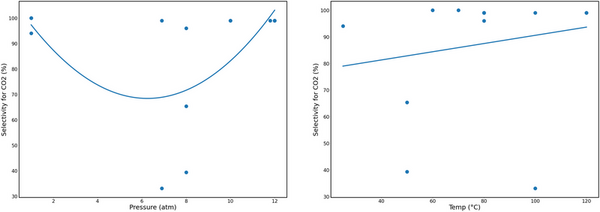
Carbon dioxide (CO2) is a major contributor to global warming, which has led to irreversible environmental and socio-economic damage. Metal-organic frameworks (MOFs) are promising new nanomaterials for use in the fight against climate change that can efficiently capture and convert CO2 to other useful carbon products, such as ethanol, methanol, and cyclic carbonates. However, the large-scale production of MOFs for industrial use is limited because of their high porosity and customizability making their development, synthesis, and use expensive. The purpose of our research was to use computational models to determine the reaction conditions under which MOFs can more efficiently capture and convert CO2. We used Python programming to examine current data presented in the literature, encompassing various MOFs with a range of pore sizes and different substrates to determine the optimal conditions for MOFs to capture and convert CO2 into useful products. Our results showed that pressure was significantly correlated in convex quadratic models with the selective capture of carbon and temperature was significantly correlated in a concave quadratic model with carbon conversion. Optimal reaction conditions were approximately 80°C (R² = 0.376) and 0-1 atm or 11-12 atm of pressure (R² = 0.210). In a cost-efficient manner, this analysis tested the hypothesis that pressure and temperature affect the efficacy of carbon capture and conversion, and contribute to understanding the optimal conditions for MOF performance to improve the use of MOFs for controlling greenhouse CO2 emissions.
This article has been tagged with: