.jpg)
In this study, potential physiological effects of Red 40 food dye, found in many different food products, are tested using Daphnia magna, a small freshwater crustacean.
Read More...The impact of Red 40 artificial food dye on the heart rate of Daphnia magna
.jpg)
In this study, potential physiological effects of Red 40 food dye, found in many different food products, are tested using Daphnia magna, a small freshwater crustacean.
Read More...The Parent-Child Relationship During the College Planning Process

To explore the parent-child relationship during college planning, authors surveyed high school juniors from two private schools (boarding school vs. non-boarding parochial school). After coding, survey answers indicate students at boarding schools were found to have greater fear of parental control and disappointment, while students at non-boarding parochial schoolexpressed a greater need for parental assistance.
Read More...A comparison of small engine emissions powered by alcohol and gasoline fuel
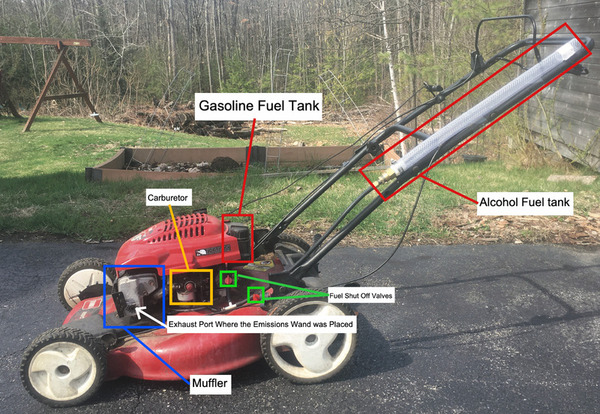
The authors looked at the emissions from a small, carbureted engine that was being powered by a mix of ethanol and methanol compared to E10 gasoline. The found that across all four pollutants measured, the ethanol-methanol mixture resulted in less emissions compared to the E10 fuel.
Read More...Optimizing airfoil shape for small, low speed, unmanned gliders: A homemade investigation

Here, the authors sought to identify a method to optimize the lift generated by an airfoil based solely on its shape. By beginning with a Bernoullian model to predict an optimized wing shape, the authors then tested their model against other possible shapes by constructing them from Styrofoam and testing them in a small wind tunnel. Contrary to their hypothesis, they found their expected optimal airfoil shape did not result in the greatest lift generation. They attributed this to a variety of confounding variables and concluded that their results pointed to a correlation between airfoil shape and lift generation.
Read More...Transfer Learning for Small and Different Datasets: Fine-Tuning A Pre-Trained Model Affects Performance
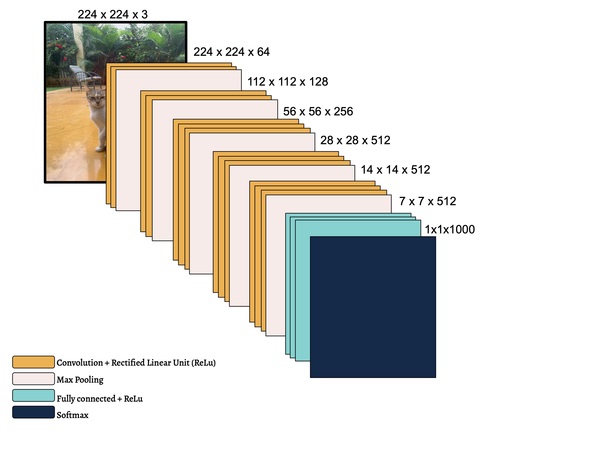
In this study, the authors seek to improve a machine learning algorithm used for image classification: identifying male and female images. In addition to fine-tuning the classification model, they investigate how accuracy is affected by their changes (an important task when developing and updating algorithms). To determine accuracy, a set of images is used to train the model and then a separate set of images is used for validation. They found that the validation accuracy was close to the training accuracy. This study contributes to the expanding areas of machine learning and its applications to image identification.
Read More...Thermoelectric cooling in greenhouses: Implications for small-holder production
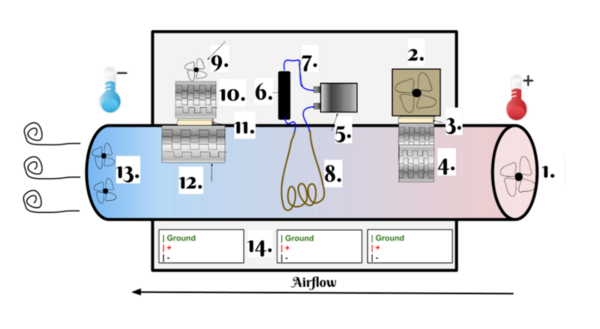
The authors set to test a system that would help with the dehumidification and overall management of greehouses.
Read More...siRNA-dependent KCNMB2 silencing inhibits lung cancer cell proliferation and promotes cell death
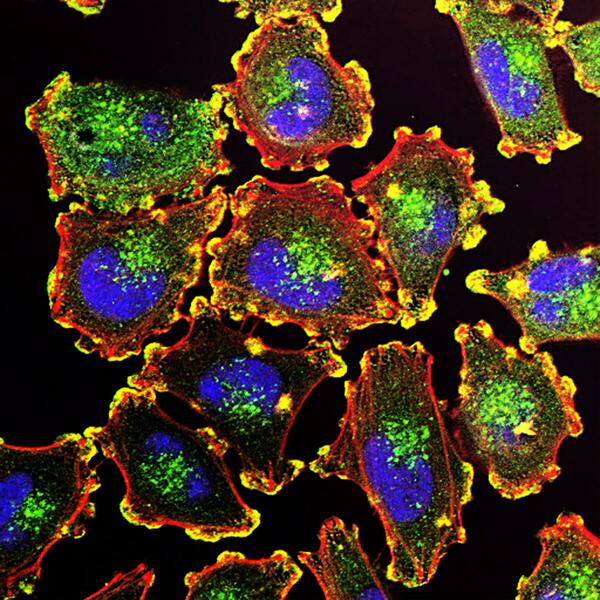
Here, seeking to better understand the genetic associations underlying non-small cell lung cancer, the authors screened hundreds of genes, identifying that KCNMB2 upregulation was significantly correlated with poor prognoses in lung cancer patients. Based on this, they used small interfering RNA to decrease the expression of KCNMB2 in A549 lung cancer cells, finding decreased cell proliferation and increased lung cancer cell death. They suggest this could lead to a new potential target for lung cancer therapies.
Read More...Understanding the Relationship Between Perception and Reality Related to Public Safety: A Case Study of Public Opinion on Local Law Enforcement in Andover, MA

A small town with low crime rates and relatively high education levels usually gives the perception of a safe and comfortable community. This study explored the connection between this perception, as defined by public opinion, and reality, as defined by US Census and FBI crime data.
Read More...Nonthermal nitrogen fixation with air and water by using a low-pressure plasma
The effect of Poisson sprinkling methods on causal sets in 1+1-dimensional flat spacetime
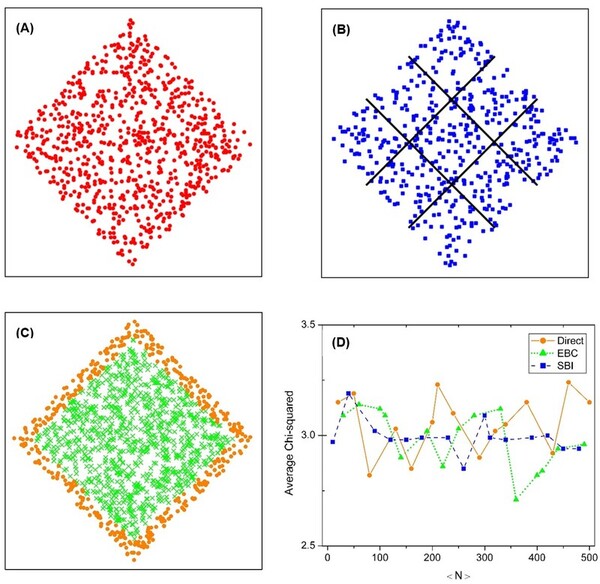
The causal set theory (CST) is a theory of the small-scale structure of spacetime, which provides a discrete approach to describing quantum gravity. Studying the properties of causal sets requires methods for constructing appropriate causal sets. The most commonly used approach is to perform a random sprinkling. However, there are different methods for sprinkling, and it is not clear how each commonly used method affects the results. We hypothesized that the methods would be statistically equivalent, but that some noticeable differences might occur, such as a more uniform distribution for the sub-interval sprinkling method compared to the direct sprinkling and edge bias compensation methods. We aimed to assess this hypothesis by analyzing the results of three different methods of sprinkling. For our analysis, we calculated distributions of the longest path length, interval size, and paths of various lengths for each sprinkling method. We found that the methods were statistically similar. However, one of the methods, sub-interval sprinkling, showed some slight advantages over the other two. These findings can serve as a point of reference for active researchers in the field of causal set theory, and is applicable to other research fields working with similar graphs.
Read More...