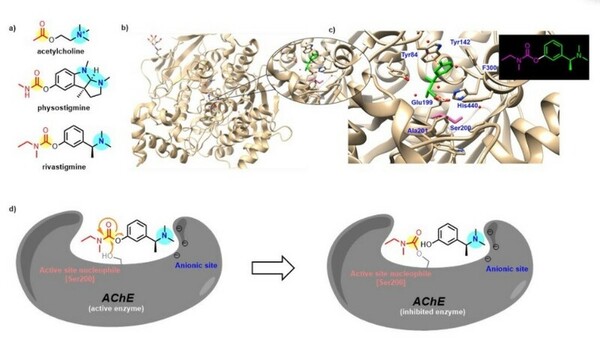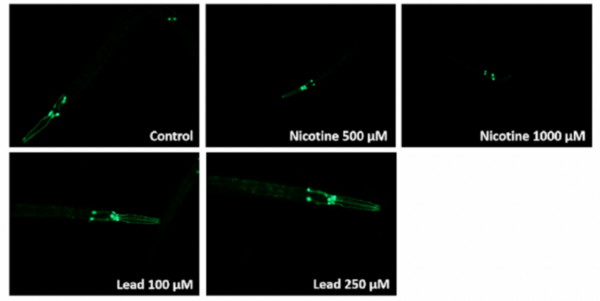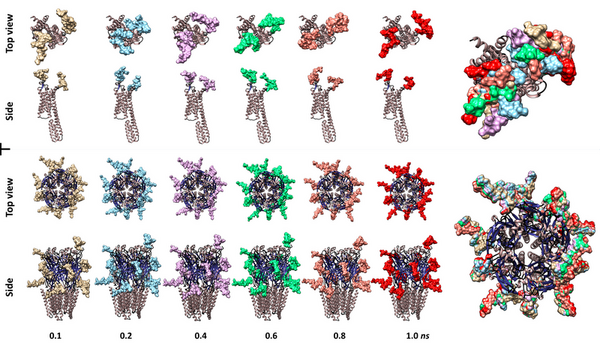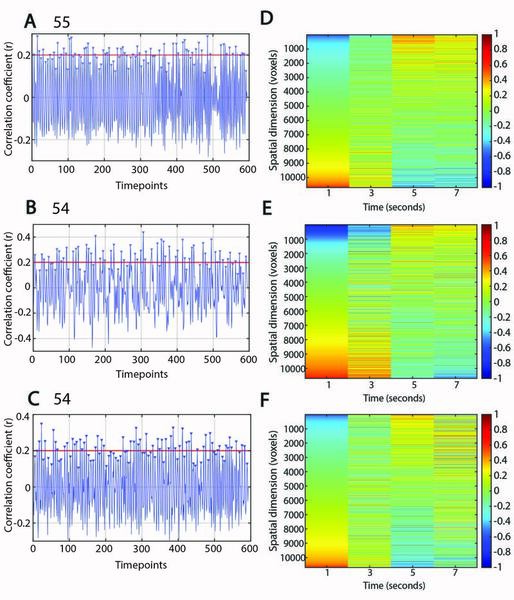
PDE8, a type of phosphodiesterase (PDE), is proven to be crucial in various cellular activities and physiological activities by influencing second messenger systems. It is involved in a wide range of diseases, including Alzheimer’s disease and various heart diseases. However, there is limited information about PDE8 selective inhibitors. This work aimed to improve the solubility and yield of PDE8 in the supernatant by exploring suitable culture conditions, including temperatures and different additives.
Read More...