Spectrophotometric comparison of 4-Nitrophenyl carbonates & carbamates as base-labile protecting groups
(1) Leigh High School, San Jose, CA, (2) BASIS Independent Silicon Valley, San Jose, CA, (3) Carlmont High School, Belmont, CA, (4) Department of Chemistry, Biochemistry, & Physical Science, Aspiring Scholars Directed Research Program, Fremont, CA
https://doi.org/10.59720/22-075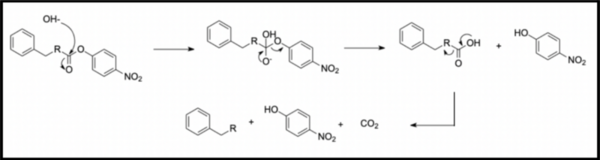
In organic synthesis, protecting groups are derivatives of reactive functionalities that play a key role in ensuring chemoselectivity of chemical transformations. To protect alcohols and amines, acid-labile tert-butyloxycarbonyl protecting groups are often employed but are avoided when the substrate is acid-sensitive. Thus, orthogonal base-labile protecting groups have been in demand to enable selective deprotection and to preserve the reactivity of acid-sensitive substrates. To meet this demand, we present 4-nitrophenyl carbonates and carbamates as orthogonal base-labile protecting group strategies. These protecting groups are relatively stable in aqueous and acidic solution yet cleaved and irreversibly decarboxylated in mild basic conditions. We showed that deprotection can be monitored spectroscopically, as hydrolysis yields 4-nitrophenol, a bright yellow compound with an optical readout at 413 nm. Finally, we demonstrated that the use of 4-nitrophenol as an effective leaving group allows for deprotection to be carried out in mild conditions. We protected benzyl alcohol and benzylamine via acylation by 4-nitrophenyl chloroformate, yielding substrates that were subsequently subjected to hydrolysis in various pH conditions. The release of 4-nitrophenol was monitored spectroscopically and the reaction kinetics were derived from absorbance data, yielding that hydrolysis was accelerated only in basic conditions and was most effective in pH 12 and above. These results inform the feasibility of our protecting group in organic synthesis and open avenues toward new synthetic routes by broadening the spectrum of effective protecting group strategies.
This article has been tagged with: