Reactivity-informed design, synthesis, and Michael addition kinetics of C-ring andrographolide analogs
(1) BASIS independent Silicon Valley, San Jose, California, (2) Mission San Jose High School, Fremont, California, (3) Saratoga High School, Saratoga, California, (4) Leigh High School, San Jose, California, (5) Amador Valley High School, Pleasanton, California, (6) American High School, Fremont, California, (7) Irvington High School, Fremont, California, (8) Department of Chemistry, Biochemistry & Physics, Aspiring Scholars Directed Research Program, Fremont, California
https://doi.org/10.59720/21-258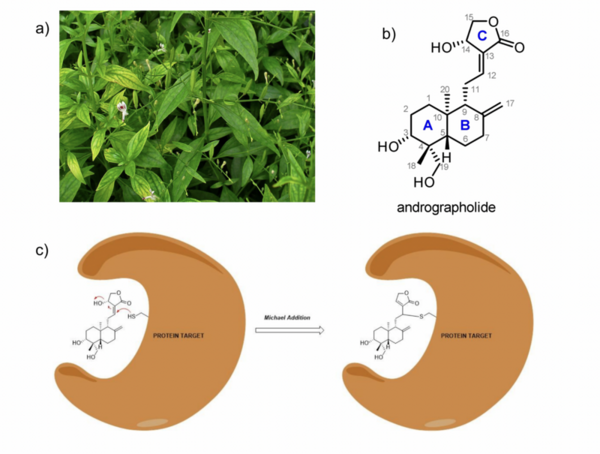
Andrographolide, a labdane diterpenoid lactone natural product extracted from Andrographis paniculata, has demonstrated potent biological activity and therapeutic potential against cancer, Alzheimer's disease, diabetes, and multiple sclerosis. Andrographolide is reported to significantly inhibit the NF-κB signaling pathway, which is active in immune system function and regulation of inflammatory cells. However, andrographolide use is not optimized for human systems. In order to improve upon these aspects, we designed and semi- synthesized a library of andrographolide analogues with modified electronics of the 12,13 unsaturated lactone to increase cytotoxicity via the addition of electron-withdrawing groups, thereby influencing reactivity of the compound. Reactivity of the top piece butenolide warhead was quantified via a time- resolved colorimetric ex vivo Michael addition assay using Elleman’s Method, wherein reduced-glutathione was the electron donor. The results demonstrate that the installation of an acetate ester at C14 did not result in greater Michael acceptor reactivity, whereas changing the electrophile to C14 with a C11-C12 alkene resulted in a significantly higher rate of reaction with glutathione. Further, computational studies were employed to model the Michael addition energetic pathways of andrographolide and each analog. We found the energetic pathways for all analogs were relatively consistent, suggesting that changes in sterics may play a more significant role in defining Michael addition kinetics. Through understanding the kinetic mechanisms of the Michael acceptor pharmacophore in andrographolide, we hope to further inform directed semisyntheses in order to optimize this incredibly potent natural product for clinical use.
This article has been tagged with: