Mutation of the Catalytic Cysteine in Anopheles gambiae Transglutaminase 3 (AgTG3) Abolishes Plugin Crosslinking Activity without Disrupting Protein Folding Properties
(1) Ocean Lakes High School, Virginia Beach, Virginia, (2) Department of Chemistry, Yale University, New Haven, Connecticut
https://doi.org/10.59720/14-005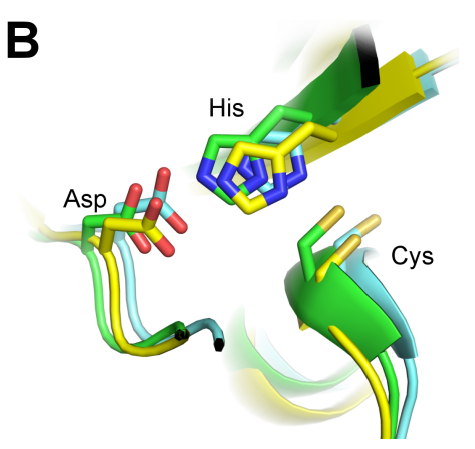
Malaria is a devastating parasitic infectious disease with an enormous impact on public health and economic growth, particularly in Sub-Saharan Africa. Besides advances in anti-malarial drugs and vaccine development, a successful malaria eradication program relies on controlling the mosquito vector. In this study, we pursue a novel approach for malaria vector control by inhibiting an enzyme important for ensuring reproductive success of the mosquito Anopheles gambiae. The enzyme, A. gambiae transglutaminase 3 (AgTG3), catalyzes the cross-linking of its native substrate Plugin, which is then transferred to a female mosquito in a coagulated mass known as the mating plug. Interfering with AgTG3-catalyzed mating plug formation prevents efficient sperm storage, with a direct consequence on fertility. This study demonstrates that the mutation of a highly conserved cysteine residue in AgTG3 (Cys323) abolishes its cross-linking activity without disrupting other properties of the enzyme such as protein folding and oligomeric assembly. These results suggest that Cys323 is an active site residue and support the design of specific inhibitors targeting this site as a promising strategy to reduce the malaria disease burden worldwide.
This article has been tagged with: