The analysis of the viral transmission and structural interactions between the HIV-1 envelope glycoprotein and the lymphocyte receptor integrin α4β7
(1) Thomas Jefferson High School for Science and Technology, Alexandria, Virginia, (2) Thomas Jefferson High School for Science and Technology, Alexandria, Virginia
https://doi.org/10.59720/20-238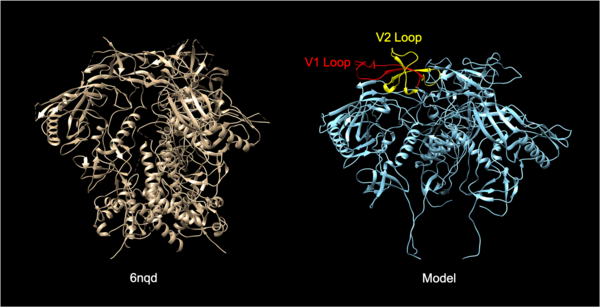
The Human Immunodeficiency Virus (HIV) infects approximately 40 million people globally, and one million people die every year from Acquired Immune Deficiency Syndrome (AIDS)-related illnesses. The HIV-1 virus proliferates, infects people, and kills those who host the virus. Unfortunately, there is little research on the structural interactions between HIV-1 proteins and human cell receptors. This study examined the interactions between the HIV-1 envelope glycoprotein gp120 and the human lymphocyte receptor integrin α4β7, the putative first long-range receptor for the envelope glycoprotein of the virus in mucosal tissues. The specific site of binding between the glycoprotein and the receptor has not been determined. However, discovering the binding site can open the field towards the research of molecules that could potentially block this interaction and prevent the initial binding of the HIV1 virus. We hypothesized that the V1 and V2 loops of the envelope glycoprotein of HIV-1 are involved in the binding between the HIV-1 virus and the human lymphocyte receptor α4β7. Using structural analysis software, we analyzed the electrostatics and structural interactions between the glycoprotein and receptor. We report structural insights into the interactions between α4β7 and envelope glycoprotein gp120. Our data support the claim that the V1 loop is involved in the binding between α4β7 and the HIV-1 envelope glycoprotein through molecular dockings.
This article has been tagged with: