Recombinant preparation and characterization of ADH1C and ALDH2 in alcohol metabolism
(1) Torrey Pines High School, (2) Shanghai Institute of Immunity and Infection, Chinese Academy of Sciences
https://doi.org/10.59720/24-135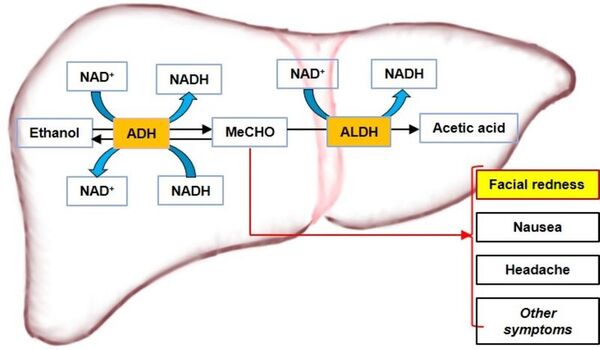
The observation that some individuals experience facial redness after consuming alcoholic beverages prompted this investigation. Alcohol metabolism, which involves the catalytic action of two key enzymes—alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH)—with NAD+/NADH as coenzymes, produces acetaldehyde (MeCHO), a toxic intermediate responsible for facial redness, nausea, and other health issues. With the increasing consumption of alcohol worldwide, alcohol-related health concerns have been drawing more attention and need to be addressed in more effective ways. We hypothesized that ADH and ALDH can be recombinantly purified and that they can detoxify alcohol in vitro. In this study, we cloned and expressed human ADH1C and ALDH2 in Escherichia coli cells and purified them using sequential chromatography. Enzymatic activity tests were performed using a microplate spectrophotometer that detected changes in NADH concentration. The results showed that both enzymes were active individually. We co-incubated ADH1C and ALDH2 with ethanol in the presence of NAD+. Gas Chromatography-Mass Spectrometry (GC-MS) detected acetic acid, and no MeCHO accumulation was observed. This confirmed that both ADH1C and ALDH2 are essential for the detoxification of alcohol in vitro. The potential applications of recombinant ADH1C and ALDH2 to detoxify alcohol await further research on an effective encapsulation strategy for delivering enzymes through the complex environments of the gastrointestinal (GI) tract.
This article has been tagged with: