The Dependence of CO2 Removal Efficiency on its Injection Speed into Water
(1) Pittsford Mendon High School
https://doi.org/10.59720/23-285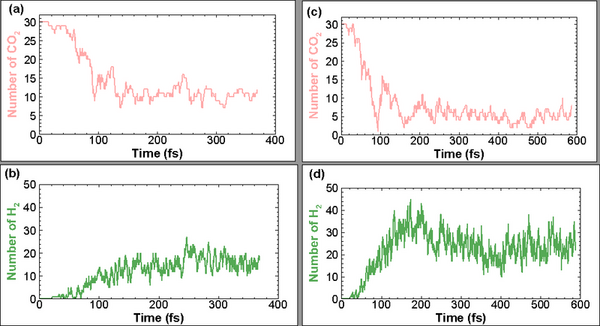
Scientific research in past decades has come to the consensus that climate change is making drastic alterations to the world we are living in today. Green-house gas emission, such as carbon dioxide (CO2) emission into Earth’s atmosphere through burning fossil fuel, is the root cause of this existential threat to humankind. Research and technological innovations are necessary to alleviate the climate crisis. This has prompted us to conduct research to find viable ways of destroying CO2, such as by breaking the bonds of the CO2 molecules through high-speed collisions. We hypothesized that injecting CO2 into liquid water could destroy the molecular bonds. Further, we investigated how fast the injection speed needs to be for effective CO2 destruction. We used a quantum molecular dynamics (QMD) method to simulate CO2 collisions with liquid water at a variety of speeds. We collected atomic and molecular positions during and after these collisions through numerical simulations. Analysis of these data suggested that more than 80% of CO2 can be destroyed if the injection speed is greater than 20 km/s, while lower injection speed is less effective in breaking up CO2. Our numerical modeling also showed an unexpected result, in that the CO2 destruction process can also produce hydrogen and CO molecules, which are clean and renewable energy sources. These findings may provide a viable path to mitigate CO2 emissions into the atmosphere, contributing to solving the urgent climate crisis.
This article has been tagged with: