Molecular Alterations in a High-Fat Mouse Model Before the Onset of Diet–Induced Nonalcoholic Fatty Liver Disease
(1) Sehwa High School, Seoul, Republic of Korea, (2) Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
https://doi.org/10.59720/16-003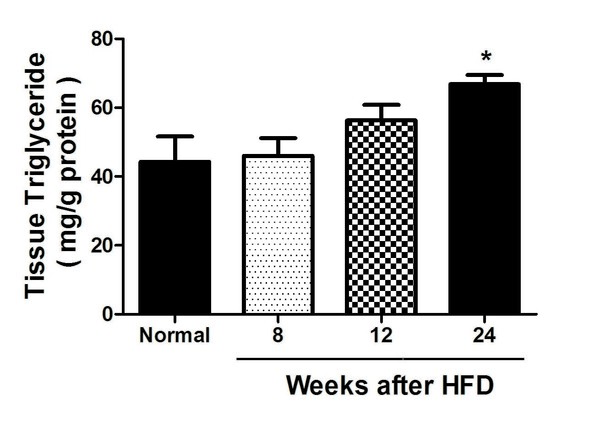
Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent chronic liver diseases worldwide. However, the precise mechanism of the disease and a definitive treatment has not yet been discovered. Studies on NAFLD frequently use an animal model fed a high-fat diet (HFD) for 24 to 48 weeks. While full-blown liver inflammation and fibrosis occur after 24 weeks of HFD administration, changes in the liver at earlier time points have not been rigorously investigated. We hypothesized that there would be some molecular alterations at an earlier time point that would predict the later onset of pathologic symptoms. The aim of our study was to delineate the changes that take place before the pathologically detectable NAFLD appears in HFD mice, which would facilitate more effective study and treatment of NAFLD. We fed C57BL/6 mice a high-fat diet for 8 to 24 weeks and evaluated liver fat accumulation, inflammation, and fibrosis. Significant pathologic fat content, inflammation, and fibrosis in the liver could only be noticed after 24 weeks of HFD. However, we observed dysregulated immune function as early as 8 weeks after the start of the diet, and detected molecular changes associated with fat accumulation by week 12. Symptoms related to liver fibrosis were the most delayed changes. Our results suggest that alterations in inflammation and fat metabolism are early changes, followed by liver fibrosis in the HFD-induced NAFLD animal model. Therefore, future NAFLD experiments using this model can be designed to include earlier time points at which the desired HFD-induced alterations occur, and NAFLD experiments that aim to investigate immunologic alterations may be performed after a shorter duration of HFD feeding.